Dynamic Nanohybrid-Polysaccharide Hydrogels for Soft Wearable Strain Sensing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Fe/TA@ChNFs Nanohybrid Synthesis
2.3. Fabrication of PVA-St-PAA-Fe/TA@ChNF Nanohybrid Hydrogels
2.4. Hydrogel Characterization
3. Results and Discussions
3.1. Design and Synthesis of PVA-St-PAA-Fe/TA@ChNF Nanohybrid Hydrogels
3.2. Mechanical, Rheological, and SELF Properties
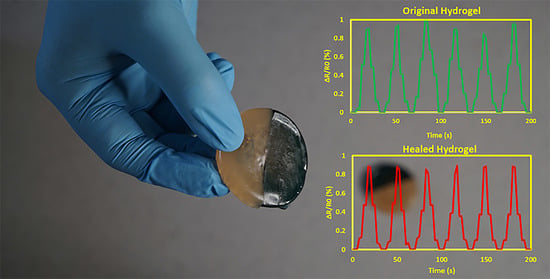
3.3. Self-Adhesiveness, Electrical Conductivity, and Strain Sensing Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heidarian, P.; Kouzani, A.Z.; Kaynak, A.; Paulino, M.; Nasri-Nasrabadi, B.; Zolfagharian, A.; Varley, R. Dynamic Plant-Derived Polysaccharide-Based Hydrogels. Carbohyd. Polym. 2019, 231, 115743. [Google Scholar] [CrossRef] [PubMed]
- Heidarian, P.; Kouzani, A.; Kaynak, A.; Paulino, M.; Nasri-Nasrabadi, B. Dynamic Hydrogels and Polymers as Inks for Three-Dimensional Printing. ACS Biomater. Sci. Eng. 2019, 5, 2688–2707. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Wang, H.; Ma, P.X.; Guo, B. Self-healing conductive hydrogels: Preparation, properties and applications. Nanoscale 2020, 12, 1224–1246. [Google Scholar] [CrossRef] [PubMed]
- Larson, C.; Peele, B.; Li, S.; Robinson, S.; Totaro, M.; Beccai, L.; Mazzolai, B.; Shepherd, R. Highly stretchable electroluminescent skin for optical signaling and tactile sensing. Science 2016, 351, 1071–1074. [Google Scholar] [CrossRef] [Green Version]
- Nasri-Nasrabadi, B.; Kaynak, A.; Heidarian, P.; Komeily-Nia, Z.; Mehrasa, M.; Salehi, H.; Kouzani, A.Z. Sodium alginate/magnesium oxide nanocomposite scaffolds for bone tissue engineering. Polym. Adv. Technol. 2018, 29, 2553–2559. [Google Scholar] [CrossRef]
- Basu, A.; Lindh, J.; Ålander, E.; Strømme, M.; Ferraz, N. On the use of ion-crosslinked nanocellulose hydrogels for wound healing solutions: Physicochemical properties and application-oriented biocompatibility studies. Carbohyd. Polym. 2017, 174, 299–308. [Google Scholar] [CrossRef]
- Zolfagharian, A.; Kouzani, A.Z.; Khoo, S.Y.; Nasri-Nasrabadi, B.; Kaynak, A. Development and analysis of a 3D printed hydrogel soft actuator. Sens. Actuator A Phys. 2017, 265, 94–101. [Google Scholar] [CrossRef]
- Taylor, D.L.; in het Panhuis, M. Self-healing hydrogels. Adv. Matter. 2016, 28, 9060–9093. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Liu, Y.-T.; Xie, X.-M. Self-healable, super tough graphene oxide–poly (acrylic acid) nanocomposite hydrogels facilitated by dual cross-linking effects through dynamic ionic interactions. J. Mater. Chem. B 2015, 3, 4001–4008. [Google Scholar] [CrossRef]
- Yang, Y.; Urban, M.W. Self-healing polymeric materials. Chem. Soc. Rev. 2013, 42, 7446–7467. [Google Scholar] [CrossRef]
- Talebian, S.; Mehrali, M.; Taebnia, N.; Pennisi, C.P.; Kadumudi, F.B.; Foroughi, J.; Hasany, M.; Nikkhah, M.; Akbari, M.; Orive, G. Self-Healing Hydrogels: The Next Paradigm Shift in Tissue Engineering? Adv. Sci. 2019, 6, 1801664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef] [PubMed]
- Mohammadinejad, R.; Maleki, H.; Larrañeta, E.; Fajardo, A.R.; Nik, A.B.; Shavandi, A.; Sheikhi, A.; Ghorbanpour, M.; Farokhi, M.; Govindh, P.; et al. Status and future scope of plant-based green hydrogels in biomedical engineering. Appl. Matter. Today 2019, 16, 213–246. [Google Scholar] [CrossRef] [Green Version]
- Nadgorny, M.; Ameli, A. Functional polymers and nanocomposites for 3D printing of smart structures and devices. ACS Appl. Mater. Interfaces 2018, 10, 17489–17507. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, J.; Qiu, C.; Xu, X.; Jin, Z. A Dual Cross-Linked Strategy to Construct Moldable Hydrogels with High Stretchability, Good Self-Recovery, and Self-Healing Capability. J. Agric. Food Chem. 2019, 67, 3966–3980. [Google Scholar] [CrossRef]
- Heidarian, P.; Behzad, T.; Sadeghi, M.J.C. Investigation of cross-linked PVA/starch biocomposites reinforced by cellulose nanofibrils isolated from aspen wood sawdust. Cellulose 2017, 24, 3323–3339. [Google Scholar] [CrossRef]
- Baghbadorani, N.B.; Behzad, T.; Etesami, N.; Heidarian, P. Removal of Cu2+ ions by cellulose nanofibers-assisted starch-g-poly (acrylic acid) superadsorbent hydrogels. Compos. B. Eng. 2019, 176, 107084. [Google Scholar] [CrossRef]
- Jayakumar, R.; Nwe, N.; Tokura, S.; Tamura, H. Sulfated chitin and chitosan as novel biomaterials. Int. J. Biol. Macromol. 2007, 40, 175–181. [Google Scholar] [CrossRef]
- Dutta, P.K.; Dutta, J.; Tripathi, V. Chitin and chitosan: Chemistry, properties and applications. J. Sci. Ind. Res. 2004, 63, 20–31. [Google Scholar]
- Hejazi, M.; Behzad, T.; Heidarian, P.; Nasri-Nasrabadi, B. A study of the effects of acid, plasticizer, cross-linker, and extracted chitin nanofibers on the properties of chitosan biofilm. Compos. Part A Appl. Sci. Manuf. 2018, 109, 221–231. [Google Scholar] [CrossRef]
- Heidarian, P.; Kouzani, A.Z.; Kaynak, A.; Paulino, M.; Nasri-Nasrabadi, B.; Varley, R. Double dynamic cellulose nanocomposite hydrogels with environmentally adaptive self-healing and pH-tuning properties. Cellulose 2019, 27, 1407–1422. [Google Scholar] [CrossRef]
- Fan, H.; Wang, J.; Jin, Z.J.M. Tough, Swelling-Resistant, Self-Healing, and Adhesive Dual-Cross-Linked Hydrogels Based on Polymer–Tannic Acid Multiple Hydrogen Bonds. Macromolecules 2018, 51, 1696–1705. [Google Scholar] [CrossRef]
- Hussain, I.; Sayed, S.M.; Liu, S.; Oderinde, O.; Yao, F.; Fu, G. Glycogen-based self-healing hydrogels with ultra-stretchable, flexible, and enhanced mechanical properties via sacrificial bond interactions. Int. J. Biol. Macromol. 2018, 117, 648–658. [Google Scholar] [CrossRef]
- Shao, C.; Wang, M.; Meng, L.; Chang, H.; Wang, B.; Xu, F.; Yang, J.; Wan, P. Mussel-inspired cellulose nanocomposite tough hydrogels with synergistic self-healing, adhesive, and strain-sensitive properties. Chem. Mater. 2018, 30, 3110–3121. [Google Scholar] [CrossRef]
- Fan, H.; Wang, J.; Zhang, Q.; Jin, Z. Tannic acid-based multifunctional hydrogels with facile adjustable adhesion and cohesion contributed by polyphenol supramolecular chemistry. ACS Omega 2017, 2, 6668–6676. [Google Scholar] [CrossRef]
- Sahiner, N.; Butun Sengel, S.; Yildiz, M. A facile preparation of donut-like supramolecular tannic acid-Fe (III) composite as biomaterials with magnetic, conductive, and antioxidant properties. J. Coord. Chem. 2017, 70, 3619–3632. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heidarian, P.; Yousefi, H.; Kaynak, A.; Paulino, M.; Gharaie, S.; Varley, R.J.; Kouzani, A.Z. Dynamic Nanohybrid-Polysaccharide Hydrogels for Soft Wearable Strain Sensing. Sensors 2021, 21, 3574. https://doi.org/10.3390/s21113574
Heidarian P, Yousefi H, Kaynak A, Paulino M, Gharaie S, Varley RJ, Kouzani AZ. Dynamic Nanohybrid-Polysaccharide Hydrogels for Soft Wearable Strain Sensing. Sensors. 2021; 21(11):3574. https://doi.org/10.3390/s21113574
Chicago/Turabian StyleHeidarian, Pejman, Hossein Yousefi, Akif Kaynak, Mariana Paulino, Saleh Gharaie, Russell J. Varley, and Abbas Z. Kouzani. 2021. "Dynamic Nanohybrid-Polysaccharide Hydrogels for Soft Wearable Strain Sensing" Sensors 21, no. 11: 3574. https://doi.org/10.3390/s21113574