Shear Stress-Dependent Targeting Efficiency Using Self-Assembled Gelatin–Oleic Nanoparticles in a Biomimetic Microfluidic System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of GONs, C-GONs, and PTX-GONs
2.2.1. Synthesis of GOC
2.2.2. Preparation of GONs Using a Desolvation Method
2.2.3. Preparation of C-GONs and PTX-GONs
2.3. Physical Characterization of GONs, C-GONs, and PTX-GONs
2.3.1. Particle Size and Surface Charge Measurements of the NPs
2.3.2. Electron Microscopy of the NPs
2.3.3. Drug Loading Content (DC) and Encapsulation Efficiency (EE)
2.4. Cell Culture in a Microfluidic Chamber
2.5. Cellular Uptake Using a Biomimetic Microfluidic System (BMS)
2.6. Determination of Cellular Uptake with C-GONs
2.6.1. Flow Cytometry
2.6.2. Confocal Laser Scanning Microscopy
2.7. Cell Viability Assay with PTX-GONs
2.8. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties: GONs, C-GONs, and PTX-GONs
3.2. Cellular Uptake of Coumarin-6-Loaded GONs
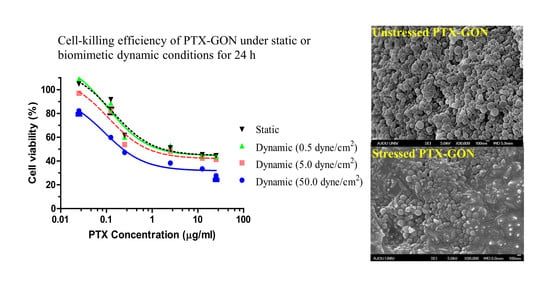
3.3. Cell-Killing Efficiency of PTX-GONs
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| NPs | Nanoparticles |
| GOC | Gelatin–oleic Conjugate |
| GON | Gelatin–oleic Nanoparticles |
| PTX-GONs | Paclitaxel-Loaded GONs |
| C-GONs | Coumarin-6-Loaded GONs |
| TEM | Transmission Electron Microscopy |
| SEM | Scanning Electron Microscopy |
| DC | Drug Loading Content |
| EE | Encapsulation Efficiency |
| BMS | Biomimetic Microfluidic System |
| IC50 | Inhibitory Concentration |
References
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Thapa, R.K.; Yong, C.S.; Kim, J.O. Nanoparticle-based combination drug delivery systems for synergistic cancer treatment. J. Pharm. Investig. 2016, 46, 325–339. [Google Scholar] [CrossRef]
- Al-azzawi, S.; Masheta, D. Designing a drug delivery system for improved tumor treatment and targeting by functionalization of a cell-penetrating peptide. J. Pharm. Investig. 2019, 49, 643–654. [Google Scholar] [CrossRef]
- Choi, Y.H.; Han, H.-K. Nanomedicines: Current status and future perspectives in aspect of drug delivery and pharmacokinetics. J. Pharm. Investig. 2019, 49, 201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, I.S.; Yoon, M.S.; Park, C.-W.; Hong, J.T.; Chung, Y.B.; Kim, J.-S.; Shin, D.H. Replacement techniques to reduce animal experiments in drug and nanoparticle development. J. Pharm. Investig. 2020, 50, 327–335. [Google Scholar] [CrossRef]
- Hare, J.I.; Lammers, T.; Ashford, M.B.; Puri, S.; Storm, G.; Barry, S.T. Challenges and strategies in anti-cancer nanomedicine development: An industry perspective. Adv. Drug Deliv. Rev. 2017, 108, 25–38. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, D.C.; Araujo, T.L.S.; Laurindo, F.R.M.; Tanaka, L.Y. Chapter 7—Hemodynamic Forces in the Endothelium: From Mechanotransduction to Implications on Development of Atherosclerosis. In Endothelium and Cardiovascular Diseases; Da Luz, P.L., Libby, P., Chagas, A.C.P., Laurindo, F.R.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 85–95. [Google Scholar] [CrossRef]
- Kang, T.; Tran, T.T.-T.; Park, C.; Lee, B.-J. Biomimetic shear stress and nanoparticulate drug delivery. J. Pharm. Investig. 2017, 47, 133–139. [Google Scholar] [CrossRef]
- Swartz, M.A.; Lund, A.W. Lymphatic and interstitial flow in the tumour microenvironment: Linking mechanobiology with immunity. Nat. Rev. Cancer 2012, 12, 210–219. [Google Scholar] [CrossRef]
- Kona, S.; Dong, J.F.; Liu, Y.; Tan, J.; Nguyen, K.T. Biodegradable nanoparticles mimicking platelet binding as a targeted and controlled drug delivery system. Int. J. Pharm. 2012, 423, 516–524. [Google Scholar] [CrossRef] [Green Version]
- Fujiwara, T.; Akita, H.; Furukawa, K.; Ushida, T.; Mizuguchi, H.; Harashima, H. Impact of convective flow on the cellular uptake and transfection activity of lipoplex and adenovirus. Biol. Pharm. Bull. 2006, 29, 1511–1515. [Google Scholar] [CrossRef] [Green Version]
- Teo, B.M.; van der Westen, R.; Hosta-Rigau, L.; Stadler, B. Cell response to PEGylated poly(dopamine) coated liposomes considering shear stress. Biochim. Biophys. Acta 2013, 1830, 4838–4847. [Google Scholar] [CrossRef] [PubMed]
- Hosta-Rigau, L.; Stadler, B. Shear stress and its effect on the interaction of myoblast cells with nanosized drug delivery vehicles. Mol. Pharm. 2013, 10, 2707–2712. [Google Scholar] [CrossRef] [PubMed]
- Meghani, N.; Kim, K.H.; Kim, S.H.; Lee, S.H.; Choi, K.H. Evaluation and live monitoring of pH-responsive HSA-ZnO nanoparticles using a lung-on-a-chip model. Arch. Pharm. Res. 2020, 43, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Liu, J.; Wang, X.; Feng, L.; Wu, J.; Zhu, X.; Wen, W.; Gong, X. Organ-on-a-chip: Recent breakthroughs and future prospects. Biomed. Eng. Online 2020, 19, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.; Vo, C.L.; Kang, T.; Oh, E.; Lee, B.-J. New method and characterization of self-assembled gelatin-oleic nanoparticles using a desolvation method via carbodiimide/N-hydroxysuccinimide (EDC/NHS) reaction. Eur. J. Pharm. Biopharm. 2015, 89, 365–373. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; Samy, W.M.; Elgindy, N.A. Protein-based nanocarriers as promising drug and gene delivery systems. J. Control. Release 2012, 161, 38–49. [Google Scholar] [CrossRef]
- Meghani, N.M.; Amin, H.H.; Park, C.; Park, J.-B.; Cui, J.-H.; Cao, Q.-R.; Lee, B.-J. Design and evaluation of clickable gelatin-oleic nanoparticles using fattigation-platform for cancer therapy. Int. J. Pharm. 2018, 545, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Park, C.; Meghani, N.M.; Tran, T.T.D.; Tran, P.H.L.; Park, J.-B.; Lee, B.-J. Utilization of a fattigation platform gelatin-oleic acid sodium salt conjugate as a novel solubilizing adjuvant for poorly water-soluble drugs via self-assembly and nanonization. Int. J. Pharm. 2020, 575, 118892. [Google Scholar] [CrossRef]
- Tran, P.H.; Tran, T.T.; Vo, T.V.; Vo, C.L.; Lee, B.-J. Novel multifunctional biocompatible gelatin-oleic acid conjugate: Self-assembled nanoparticles for drug delivery. J. Biomed. Nanotechnol. 2013, 9, 1416–1431. [Google Scholar] [CrossRef]
- Singh, D.; Bedi, N.; Tiwary, A.K. Enhancing solubility of poorly aqueous soluble drugs: Critical appraisal of techniques. J. Pharm. Investig. 2017, 48, 509–526. [Google Scholar] [CrossRef]
- Park, C.; Meghani, N.M.; Amin, H.H.; Nguyen, V.H.; Lee, B.-J. Patient-centered drug delivery and its potential applications for unmet medical needs. Ther. Deliv. 2017, 8, 775–790. [Google Scholar] [CrossRef]
- Park, C.; Meghani, N.; Loebenberg, R.; Cui, J.-H.; Cao, Q.-R.; Lee, B.-J. Fatty acid chain length impacts nanonizing capacity of albumin-fatty acid nanomicelles: Enhanced physicochemical property and cellular delivery of poorly water-soluble drug. Eur. J. Pharm. Biopharm. 2020, 152, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.; Cho, Y.; Park, C.; Kim, S.D.; Oh, E.; Cui, J.-H.; Cao, Q.-R.; Lee, B.-J. Effect of biomimetic shear stress on intracellular uptake and cell-killing efficiency of doxorubicin in a free and liposomal formulation. Int. J. Pharm. 2016, 510, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.; Park, C.; Choi, J.S.; Cui, J.-H.; Lee, B.-J. Effects of shear stress on the cellular distribution of polystyrene nanoparticles in a biomimetic microfluidic system. J. Drug Deliv. Sci. Technol. 2016, 31, 130–136. [Google Scholar] [CrossRef]
- Khan, S.A.; Schneider, M. Improvement of nanoprecipitation technique for preparation of gelatin nanoparticles and potential macromolecular drug loading. Macromol. Biosci. 2013, 13, 455–463. [Google Scholar] [CrossRef]
- Won, Y.W.; Kim, Y.H. Recombinant human gelatin nanoparticles as a protein drug carrier. J. Control. Release 2008, 127, 154–161. [Google Scholar] [CrossRef]
- Lee, S.; Lee, C.; Kim, B.; Lee, E.S.; Kim, J.O.; Oh, K.T.; Choi, H.-G.; Youn, Y.S. A novel prototype of albumin nanoparticles fabricated by supramolecular cyclodextrin-adamantane association. Colloids Surf. B Biointerfaces 2016, 147, 281–290. [Google Scholar] [CrossRef]
- Tee, J.K.; Yip, L.X.; Tan, E.S.; Santitewagun, S.; Prasath, A.; Ke, P.C.; Ho, H.K.; Leong, D.T. Nanoparticles’ interactions with vasculature in diseases. Chem. Soc. Rev. 2019, 48, 5381–5407. [Google Scholar] [CrossRef]
- Huang, Q.; Hu, X.; He, W.; Zhao, Y.; Hao, S.; Wu, Q.; Li, S.; Zhang, S.; Shi, M. Fluid shear stress and tumor metastasis. Am. J. Cancer Res. 2018, 8, 763–777. [Google Scholar]
- Tseng, C.L.; Wang, T.W.; Dong, G.C.; Wu, S.Y.H.; Young, T.H.; Shieh, M.J.; Lou, P.J.; Lin, F.H. Development of gelatin nanoparticles with biotinylated EGF conjugation for lung cancer targeting. Biomaterials 2007, 28, 3996–4005. [Google Scholar] [CrossRef]
- Jahanshahi, M.; Babaei, Z. Protein nanoparticle: A unique system as drug delivery vehicles. Afr. J. Biotechnol. 2008, 7, 4926–4934. [Google Scholar]
- Hou, J.; Sun, E.; Zhang, Z.H.; Wang, J.; Yang, L.; Cui, L.; Ke, Z.C.; Tan, X.B.; Jia, X.B.; Lv, H. Improved oral absorption and anti-lung cancer activity of paclitaxel-loaded mixed micelles. Drug Deliv. 2017, 24, 261–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, A.; Stellacci, F. Effect of surface properties on nanoparticle-cell interactions. Small 2010, 6, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Ruzycka, M.; Cimpan, M.R.; Rios-Mondragon, I.; Grudzinski, I.P. Microfluidics for studying metastatic patterns of lung cancer. J. Nanobiotechnol. 2019, 17, 71. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Lane, L.A. Probing the biological obstacles of nanomedicine with gold nanoparticles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1542. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Lee, B.-J. Protein corona: A new approach for nanomedicine design. Int. J. Nanomed. 2017, 12, 3137–3151. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, V.H.; Meghani, N.M.; Amin, H.H.; Tran, T.T.D.; Tran, P.H.L.; Park, C.; Lee, B.-J. Modulation of serum albumin protein corona for exploring cellular behaviors of fattigation-platform nanoparticles. Colloids Surf. B Biointerfaces 2018, 170, 179–186. [Google Scholar] [CrossRef]
- Foroozandeh, P.; Aziz, A.A. Insight into Cellular Uptake and Intracellular Trafficking of Nanoparticles. Nanoscale Res. Lett. 2018, 13, 339. [Google Scholar] [CrossRef]
- Fleischer, C.C.; Payne, C.K. Nanoparticle-cell interactions: Molecular structure of the protein corona and cellular outcomes. Acc. Chem. Res. 2014, 47, 2651–2659. [Google Scholar] [CrossRef]
- DeVerse, J.S.; Sandhu, A.S.; Mendoza, N.; Edwards, C.M.; Sun, C.; Simon, S.I.; Passerini, A.G. Shear stress modulates VCAM-1 expression in response to TNF-alpha and dietary lipids via interferon regulatory factor-1 in cultured endothelium. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H1149–H1157. [Google Scholar] [CrossRef] [Green Version]
- Regmi, S.; Fu, A.; Luo, K.Q. High shear stresses under exercise condition destroy circulating tumor cells in a microfluidic system. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Rana, K.; Liesveld, J.L.; King, M.R. Delivery of apoptotic signal to rolling cancer cells: A novel biomimetic technique using immobilized TRAIL and E-selectin. Biotechnol. Bioeng. 2009, 102, 1692–1702. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Mitragotri, S. Impact of particle elasticity on particle-based drug delivery systems. Adv. Drug Deliv. Rev. 2017, 108, 51–67. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Zhang, M.; Kumar, S.; Vogus, D.R.; Menegatti, S.; Helgeson, M.E.; Mitragotri, S. Elasticity of nanoparticles influences their blood circulation, phagocytosis, endocytosis, and targeting. ACS Nano 2015, 9, 3169–3177. [Google Scholar] [CrossRef] [PubMed]
- Dukette, T.E.; Mackay, M.E.; Van Horn, B.; Wooley, K.L.; Drockenmuller, E.; Malkoch, M.; Hawker, C.J. Conformation of intramolecularly cross-linked polymer nanoparticles on solid substrates. Nano Lett. 2005, 5, 1704–1709. [Google Scholar] [CrossRef]
- Wirtz, D.; Konstantopoulos, K.; Searson, P.C. The physics of cancer: The role of physical interactions and mechanical forces in metastasis. Nat. Rev. Cancer 2011, 11, 512–522. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, M.J.; King, M.R. Computational and experimental models of cancer cell response to fluid shear stress. Front. Oncol. 2013, 3, 44. [Google Scholar] [CrossRef] [Green Version]
- Shang, M.; Soon, R.H.; Lim, C.T.; Khoo, B.L.; Han, J. Microfluidic modelling of the tumor microenvironment for anti-cancer drug development. Lab Chip 2019, 19, 369–386. [Google Scholar] [CrossRef]







| Type of NPs | Particle Size (nm) | PDI * | Zeta Potential (mV) |
|---|---|---|---|
| GONs | 163.23 ± 7.91 | 0.105 ± 0.03 | −64.62 ± 1.37 |
| C-GONs | 199.07 ± 1.48 | 0.164 ± 0.03 | −36.11 ± 8.28 |
| PTX-GONs | 309.21 ± 3.56 | 0.064 ± 0.05 | −23.73 ± 1.18 |
| PTX-GONs after 50 dynes/cm2 | 291.48 ± 4.72 | 0.512 ± 0.08 | −21.44 ± 3.51 |
| Cell Culture Conditions | IC50 Values (µg/mL) of PTX-GONs |
|---|---|
| Static condition | 0.1377 ± 0.0622 |
| Dynamic condition at 0.5 dynes/cm2 | 0.1057 ± 0.0474 |
| Dynamic condition at 5 dynes/cm2 | 0.1084 ± 0.0584 |
| Dynamic condition at 50 dynes/cm2 | 0.0914 ± 0.0465 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, T.; Park, C.; Meghani, N.; Tran, T.T.D.; Tran, P.H.L.; Lee, B.-J. Shear Stress-Dependent Targeting Efficiency Using Self-Assembled Gelatin–Oleic Nanoparticles in a Biomimetic Microfluidic System. Pharmaceutics 2020, 12, 555. https://doi.org/10.3390/pharmaceutics12060555
Kang T, Park C, Meghani N, Tran TTD, Tran PHL, Lee B-J. Shear Stress-Dependent Targeting Efficiency Using Self-Assembled Gelatin–Oleic Nanoparticles in a Biomimetic Microfluidic System. Pharmaceutics. 2020; 12(6):555. https://doi.org/10.3390/pharmaceutics12060555
Chicago/Turabian StyleKang, Taehee, Chulhun Park, Nileshkumar Meghani, Thao T.D. Tran, Phuong H.L. Tran, and Beom-Jin Lee. 2020. "Shear Stress-Dependent Targeting Efficiency Using Self-Assembled Gelatin–Oleic Nanoparticles in a Biomimetic Microfluidic System" Pharmaceutics 12, no. 6: 555. https://doi.org/10.3390/pharmaceutics12060555